from IPython.core.display import HTML
def _set_css_style(css_file_path):
"""
Read the custom CSS file and load it into Jupyter.
Pass the file path to the CSS file.
"""
styles = open(css_file_path, "r").read()
s = '<style>%s</style>' % styles
return HTML(s)
_set_css_style('rise.css')
Sequence analysis and biopython¶
- Sequence data and formats
- Sequence objects in Biopython
- Sequence search
- Alignment objects in Biopython
FASTA¶
First line is description of sequence and starts with >
All lines up to the next > are part of the same sequence
Usually less than 80 characters per line
>gi|568815581:c4949086-4945650 Homo sapiens chromosome 17, GRCh38.p2 Primary Assembly
CCCGCAGGGTCCACACGGGTCGGGCCGGGCGCGCTCCCGTGCAGCCGGCTCCGGCCCCGACCGCCCCATG
CACTCCCGGCCCCGGCGCAGGCGCAGGCGCGGGCACACGCGCCGCCGCCCGCCGGTCCTTCCCTTCGGCG
GAGGTGGGGGAAGGAGGAGTCATCCCGTTTAACCCTGGGCTCCCCGAACTCTCCTTAATTTGCTAAATTT
GCAGCTTGCTAATTCCTCCTGCTTTCTCCTTCCTTCCTTCTTCTGGCTCACTCCCTGCCCCGATACCAAA
GTCTGGTTTATATTCAGTGCAAATTGGAGCAAACCCTACCCTTCACCTCTCTCCCGCCACCCCCCATCCT
TCTGCATTGCTTTCCATCGAACTCTGCAAATTTTGCAATAGGGGGAGGGATTTTTAAAATTGCATTTGCA
Genbank¶
Annotated format. Starts with LOCUS field. Can have several other annotation (e.g. KEYWORDS, SOURCE, REFERENCE, FEATURES).
ORIGIN record indicates start of sequence
'\\' indicates the end of sequence
LOCUS CAG28598 140 aa linear PRI 16-OCT-2008
DEFINITION PFN1, partial [Homo sapiens].
ACCESSION CAG28598
VERSION CAG28598.1 GI:47115277
DBSOURCE embl accession CR407670.1
KEYWORDS .
SOURCE Homo sapiens (human)
ORGANISM Homo sapiens
Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Euteleostomi;
Mammalia; Eutheria; Euarchontoglires; Primates; Haplorrhini;
Catarrhini; Hominidae; Homo.
ORIGIN
1 magwnayidn lmadgtcqda aivgykdsps vwaavpgktf vnitpaevgv lvgkdrssfy
61 vngltlggqk csvirdsllq dgefsmdlrt kstggaptfn vtvtktdktl vllmgkegvh
121 gglinkkcye mashlrrsqy
//
Biopython¶
Biopython features include parsers for bioinformatics file formats (BLAST, Clustalw, FASTA, Genbank,...), access to online services (NCBI, Expasy,...), interfaces to common and not-so-common programs (Clustalw, DSSP, MSMS...), a standard sequence class, various clustering modules, a KD tree data structure, and even documentation.
Other modules that might be of interest:
- Pycogent: http://pycogent.org/
- bx-python: http://bitbucket.org/james_taylor/bx-python/wiki/Home
- DendroPy: http://packages.python.org/DendroPy/
- Pygr: http://code.google.com/p/pygr/
- bioservices: https://bioservices.readthedocs.io/en/master/
Biopython is not for performing sequencing itself (see: Pitt CRC workshops).
Sequence Objects¶
from Bio.Seq import Seq # the submodule structure of biopython is a little awkward
s = Seq('GATTACA')
s
Sequences act a lot like strings, but have additional methods
Methods shared with str: count, endswith, find, lower, lstrip, rfind, split, startswith, strip, upper
Seq methods:back_transcribe, complement, reverse_complement, tomutable, tostring, transcribe, translate, ungap
Accessing Seq data¶
Sequences act like strings (indexed from 0)
s[0]
s[2:4] # returns sequence
s.lower()
s + s
The Central Dogma¶
DNA coding strand (aka Crick strand, strand +1)
5’ ATGGCCATTGTAATGGGCCGCTGAAAGGGTGCCCGATAG 3’
|||||||||||||||||||||||||||||||||||||||
3’ TACCGGTAACATTACCCGGCGACTTTCCCACGGGCTATC 5’
DNA template strand (aka Watson strand, strand −1)
|
Transcription
↓
5’ AUGGCCAUUGUAAUGGGCCGCUGAAAGGGUGCCCGAUAG 3’
Single stranded messenger RNA
|
Translation
↓
MAIVMGR*KGAR*
amino acid sequence (w/stop codons)
dna = Seq('GATTACAGATTACAGATTACA')
dna.complement(),dna.reverse_complement()
The Central Dogma¶
dna
rna = dna.transcribe()
rna
protein = rna.translate()
protein
dna.translate() # unlike cells, don't actually need rna
Codon Tables¶
By default the standard translation table is used, but others can be provided to the translate method.
from Bio.Data import CodonTable
print(sorted(CodonTable.unambiguous_dna_by_name.keys()))
print(CodonTable.unambiguous_dna_by_name['Standard'])
SeqRecord¶
Sequence data is read/written as SeqRecord objects.
These objects store additional information about the sequence (name, id, description, features)
SeqIO reads sequence records:
- must specify format
readto read a file with a single recordparseto iterate over file with mulitple records
from Bio.SeqRecord import SeqRecord
from Bio import SeqIO
seq = SeqIO.read('../files/p53.gb','genbank')
seq
seqs = []
# https://MSCBIO2025-2024.github.io/files/hydra.fasta
for s in SeqIO.parse('../files/hydra.fasta','fasta'):
seqs.append(s)
len(seqs)
Fetching sequences from the Internet¶
Biopython provides and interface to the NCBI "Entrez" search engine
The results of internet queries are returned as file-like objects
from Bio import Entrez
Entrez.email = 'jpbarton@pitt.edu' # biopython forces you to provide your email
res = Entrez.read(Entrez.einfo()) # the names of all available databases
res
print(sorted(res['DbList']))
ESearch¶
You can search any database for a given term and it will return the ids of all the relevant records
result = Entrez.esearch(db='nucleotide', term='tp53') # the result is a file-like object of the raw xml data
records = Entrez.read(result) # put into a more palatable form (dictionary)
print(records)
There were many hits, but by default only 20 are returned
We can change this (and other parameters) by changing our search terms
records = Entrez.read(Entrez.esearch(db='nucleotide', term='tp53', retmax=50))
records
EFetch¶
To get the full record for a given id, use efetch.
Must provide rettype (available options include fasta and gb)
retmode can be text or xml
#fetch the genbank file for the first id from our search
result = Entrez.efetch(db="nucleotide",id=records['IdList'][0],rettype="gb",retmode='text')
#parse into a seqrecord
p53 = SeqIO.read(result,'gb')
result
p53
Features¶
Genbank files are typically annotated with features, which refer to portions of the full sequence
SeqRecord objects track these features and you can extract the corresponding subsequence
CDS - coding sequence
p53.features
Extracting subsequences¶
cdsfeature = p53.features[3]
print(cdsfeature)
The subsequence the feature refers to can be extracted from the original full sequence using the feature.
coding = cdsfeature.extract(p53) #pass the full record (p53) to the feature
coding
BLAST¶
Biopython let's you use NCBI's BLAST to search for similar sequences with qblast which has three required arguments:
- program: blastn, blastp, blastx, tblastn, tblastx
- database: see website
- sequence: a sequence object
BLAST uses a heuristic approximation of the Smith-Waterman pairwise sequence alignment algorithm
from Bio.Blast import NCBIWWW
result = NCBIWWW.qblast('blastn','nt',coding.seq,hitlist_size=250)
# result is a file-like object with xml in it - it can take a while to get results
from Bio.Blast import NCBIXML #for parsing xmls
blast_records = NCBIXML.read(result)
print(len(blast_records.alignments),len(blast_records.descriptions))
alignment = blast_records.alignments[0]
print(len(alignment.hsps))
hsp = alignment.hsps[0] # high scoring segment pairs
print('****Alignment****')
print('sequence:', alignment.title)
print('length:', alignment.length)
print('e value:', hsp.expect)
print(hsp.query[0:75] + '...') # what we searched with
print(hsp.match[0:75] + '...')
print(hsp.sbjct[0:75] + '...') # what we matched to
alignment = blast_records.alignments[-1] # get last alignment
hsp = alignment.hsps[0]
print('****Alignment****')
print('sequence:', alignment.title)
print('length:', alignment.length)
print('e value:', hsp.expect)
print(hsp.query[0:75] + '...') # what we searched with
print(hsp.match[0:75] + '...')
print(hsp.sbjct[0:75] + '...') # what we matched to
Alignments¶
AlignIO is used to read alignment files (must provide format)
from Bio import AlignIO
align = AlignIO.read('../files/hydra179.aln','clustal')
align
print(align)
Slicing Alignments¶
Alignments are sliced just like numpy arrays
align[0] # first row
align[:,0] # first column
print(align[:,0:10])
And now for a brief foray into marine microbiology...¶

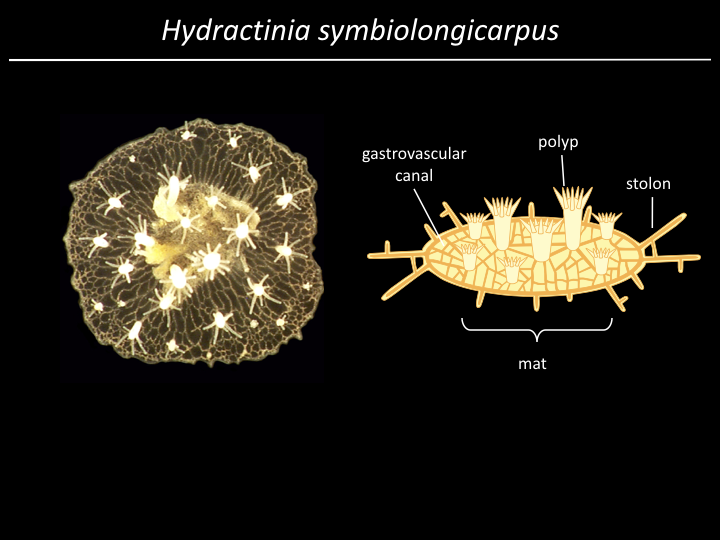
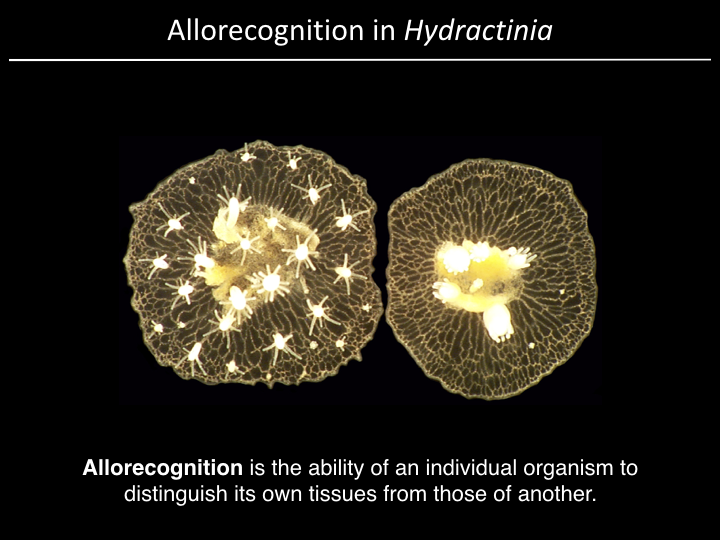

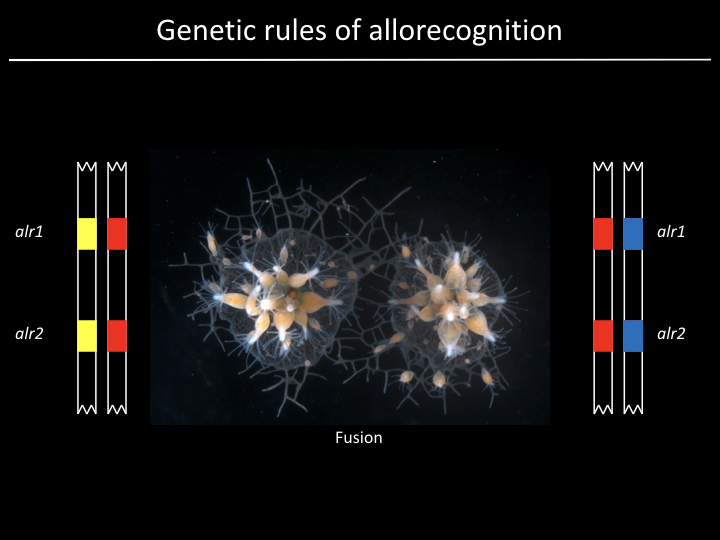
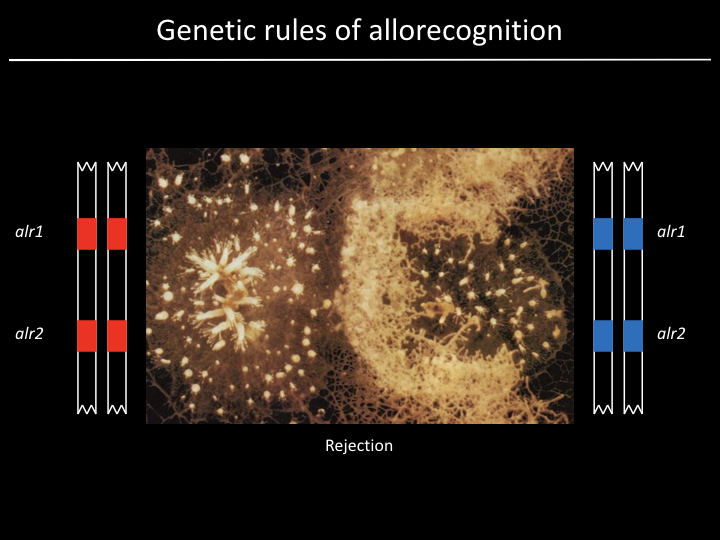
Project¶
We have a gene (alr2), but what part of the gene is responsible for allorecognition?
Given 179 sequences, how might we find out?
Find the part of the sequence that changes the most
- Count number of distinct residues at each position and plot
- Count number of distinct subsequences of length 10 at each position and plot
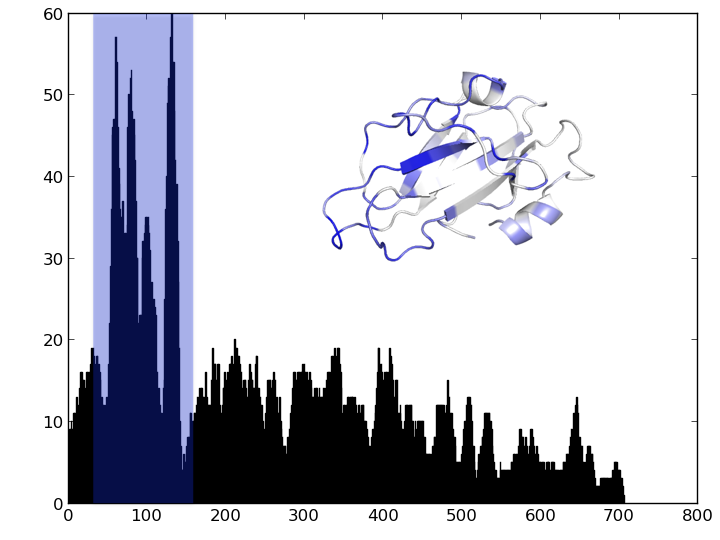
For next time¶
More sequence analysis!
Phyolgenetic trees, sequence motifs, and list comprehensions